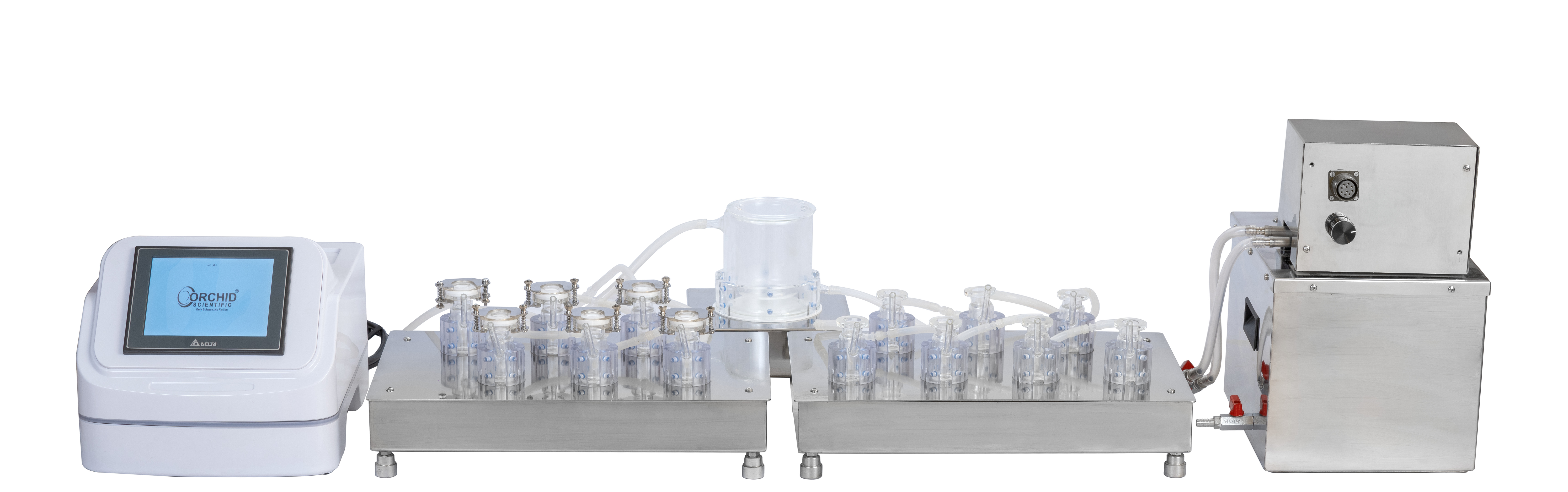
Diffusion Cell Apparatus
Model- EMFDC06 – O30
In-vitro skin permeation studies of topical formulations
The Vertical Franz Diffusion Cell is a simple, reproducible test for measuring the in vitro drug release from creams, ointments and gels.
The Franz Cell consists of two primary chambers separated by a membrane. The test product is applied to the membrane via the top chamber- donor compartment. The bottom chamber- receptor compartment contains fluid from which samples are taken at regular intervals for analysis. This testing determines the amount of active drug that has permeated the membrane at each time point.
The Transdermal Diffusion cell apparatus is remarkably simple to operate; the system is supplied with:
Six stage magnetic stirrer with digital RPM indicator
Water heater & Water circulation system with digital temperature controller and water level indicator
Cell holders
Diffusion Cells
Teflon coated stirring bars

Model- JFDC07 – O30
In-vitro skin permeation studies of topical formulations
The Vertical Franz Diffusion Cell is a simple, reproducible test for measuring the in vitro drug release from creams, ointments and gels.The Franz Cell consists of two primary chambers separated by a membrane. The test product is applied to the membrane via the top chamber- donor compartment. The bottom chamber- receptor compartment contains fluid from which samples are taken at regular intervals for analysis. This testing determines the amount of active drug that has permeated the membrane at each time point.
The Transdermal Diffusion cell apparatus is remarkably simple to operate; the system is supplied with:
Seven-stage magnetic stirrer with cell holders.
Heated circulating water bath with water level sensor.
Touch screen control unit for control of speed and temperature.
Diffusion Cells & accessories pack.
Jacked reservoir beaker for diffusion media
Performance Verification Test (PVT) for Diffusion Cell Apparatus: (Suitable for Model JFDC 07)
The Vertical Diffusion Cell (VDC) is an in vitro laboratory device for the study of drug release and permeation of semi-solid topical formulations and topical patches. Franz Diffusion Cell is a type of VDC. It is the industry standard for IVRT as well as IVPT studies. However, this Franz diffusion cell needs to pass physical acceptance criteria and performance verification test before performing IVRT/IVPT studies.
Benefits of Performance Verification Test (PVT) a USP requirement:
Globally recognized method for Metrology aspects: PVT covers both mechanical and analytical parts of franz diffusion cell apparatus.
Ability to uncover the influence of unforeseen system variables in franz diffusion cell apparatus.
Ability to compare intra/inter lab variation: PVT is a holistic test and involves system suitability testing by reference standard material (USP 1% Hydrocortisone cream), a validated reference standard cream with pre-established testing characteristics.
A necessity for IVRT/IVPT studies
PVT test will cover:
Physical Acceptance Criteria according to USP General Chapter <725> and <1724> for parameters like the capacity of cells, the diameter of the orifice, the temperature of receptor medium, the temperature of the membrane surface, and magnetic stirrer speed.
Performance Verification Test of VDC Systems using the USP Hydrocortisone Cream for parameters like Cumulative amount of drug (Hydrocortisone) release per square cm and Confidence Interval using HPLC as an analytical method.
Features
Highly accurate stirring speed and temperature controller
Precise speed control over stirring range 200-800 rpm using digital speed indicator and controller
Digital Temperature controller for precise temperature control
Water Circulation System with water level sensors to avoid damage to heating element
Magnetics Stirrer with stainless steel body to avoid corrosion
Sturdy, Heavy duty magnetic Stirrers – allowing continuous & Nonstop Operation
System Specification & Models
| Specification | Model |
EMFDC06-O30 |
|
| Stirrer Drive | |
Stirrer drive |
6 stage |
Material of construction of enclosure |
S.S. 304 |
RPM Indicator |
Digital |
RPM Range |
200-800 rpm (±1%) |
| Water circulation System | |
Material of construction of enclosure |
S.S. 304 |
Temperature range & accuracy |
5°C Above ambient to 60°C (±0.2°C) |
Order Information
| Model | Stirring drive | Certifications | Power requirements | Cells Supplied | Accessories supplied | Optional |
EMFDC06-O30 |
6 stage |
CE Compliant |
220/230V AC 50Hz
|
6 Nos cell of 20ml volume |
Clamps-6, |
|
Features
Vibration-free electromagnetic stirrer.
Data logging for stirring speed and temperature at a sampling interval.
Multi-language screen selection.
Touch screen display with 20 in-built programs.
The same system can be upgraded to multi-station by adding one more stirrer unit.
Audio/visual alarms for sampling intervals.
Stirring on/off option prior to sampling.
Highly accurate stirring speeds and temperature.
Heated circulating water bath with water level sensors to avoid damage to the heating element.
Double wall Heated circulating water bath ensuring more precise temperature control.
Password-protected data collection and report generation software with admin features.
Calibration report generation facility in the software.
Graphical presentation of data.
Provision to add experiment title & comment.
Data can be converted to Excel & Pdf files for further analysis.
System Specification & Models
| Specification | Model |
JFDC 07 (Single Stirrer Unit) |
|
| Stirrer Drive | |
Stirrer drive |
7 stage electromagnetic: 01 (JFDC 07) |
Material of construction of enclosure |
S.S. 304 |
RPM Indicator |
Digital (Touch Screen) |
Stirring Speed Range & Accuracy |
200-800 rpm (±1%) |
| Heated Circulating Water Bath (WCS02) | |
Material of construction of enclosure |
Double wall S.S. 304 |
Temperature range and accuracy |
5°C Above ambient to 60°C (±1.0 °C) |
Inter cell temperature variation |
±1.0 °C |
Inter cell stirring speed variation |
±1.0 % |
Temperature Indicator |
Digital (Touch Screen) |
Certifications |
CE Compliant |
Power requirements |
220/230V AC 50Hz, 110/120V AC 50-60Hz* |
Order Information
| Model | Stirring drive | Heated circulating water bath | Cells Supplied | Accessories supplied | Optional |
JFDC07 |
7 stage electromagnetic stirrer drive: 01 |
Double wall Heated circulating water bath: 01 unit |
Glass diffusion
cell of 20ml
capacity with
20mm
exposure area:
07Nos |
|
|
JFDC 07 x 2 (Dual stirrer unit) |
7 stage electromagnetic stirrer drive: 02 |
Double wall Heated circulating water bath: 01 unit |
Glass diffusion
cell of 20ml
capacity with
20mm
exposure area:
14 Nos |
|
Note: Orchid's continuing product development makes specifications subject to change without prior notification.
*Needs to be specified in order information
Diffusion Cell Apparatus :
1. Chauhan, M.K. and Gulati, A., 2016. AGGRANDIZED TRANSDERMAL DELIVERY OF GLIMEPIRIDE VIA TRANSFERSOMES: FORMULATION, EVALUATION AND STATISTICAL OPTIMISATION. Journal of Drug Delivery and Therapeutics, 6(4), pp.48-54.
2. K.Sumalatha*, A.Srinivasa Rao, P.Latha ,2014.DESIGN AND INVITRO EVALUATION OF NANOGE L CONTAINING MENTHA PIPERITA. Sumalatha K et al. / American Journal of Biological and Pharmaceutical Research. 2014;1(3):136-139.
3. Sherafudeen, S.P. and Vasantha, P.V., 2015. Development and evaluation of in situ nasal gel formulations of loratadine. Research in pharmaceutical sciences, 10(6), p.466.
4.Leeladurga, V., Teja, U.C., Sultana, S.A., Sudeep, K., Anusha, V.S.S., Han, T., Nalluri, B.N. and Das, D.B., 2015. Application of Microneedle Arrays for Enhancement of Transdermal Permeation of Insulin: In Vitro Experiments, Scaling Analyses and Numerical Simulations. AAPS PharmSciTech, pp.1-8.
5.Patel, R.B., Vekaria, K.B. and Patel, M.R., 2016. TLC-densitometric method for quantitation of Lurasidone hydrochloride in nanoemulsion, microemulsion, for
equilibrium solubility and ex vivo diffusion studies. Thai Journal of Pharmaceutical Sciences (TJPS), 40(1).
6.Nalluri, B.N., Ram, P.P., Teja, U.C. and Sultana, S.A., 2014. FORMULATION AND EVALUATION OF DRUG IN ADHESIVE TRANSDERMAL PATCHES OF RIVASTIGMINE. Indo American Journal of Pharmaceutical Research, 4(11), pp.5242-5248.
7.Nalluri, B.N., Kosuri, S., Valluru, S.S.A., Uppuluri, C.T. and Sultana, A., Microneedle Assisted Transdermal Delivery of Levodopa. Indian Journal of Pharmaceutical Education and Research | Vol 50 | Issue 2 | Apr-Jun, 2016
8.Arunkumar, S., Ashok, P., Desai, B.G. and Shivakumar, H.N., 2015. Effect of chemical penetration enhancer on transdermal iontophoretic delivery of diclofenac sodium under constant voltage. Journal of Drug Delivery Science and Technology, 30, pp.171-179.
9.Kumbhare, M. and Sivakumar, T., 2011. Anti-inflammatory and antinociceptive activity of pods of Caesalpinia pulcherrima. Journal of Applied Pharmaceutical Science, 1(7), p.18.
10.M.P.SINGH, B.P. NAGORI, N.R. SHAW, R. SOLANKI, M.TIWAR. , 2014. A COMPARISON STUDY OF Q.C. PARAMETERS OF MARKETED TOPICAL GEL FORMULATIONS AND RAJASTHAN GOVT. SUPPLIED FREE TOPICAL GEL FORMULATIONS. INTERNATIONAL JOURNAL OF PHARMACEUTICAL RESEARCH AND BIO-SCIENCE. JPRNK ISSN: 2277-8713; Volume 3(1):471-481
11.Jangde, R. and Singh, D., 2016. Preparation and optimization of quercetin-loaded liposomes for wound healing, using response surface methodology. Artificial cells, nanomedicine, and biotechnology, 44(2), pp.635-641.
12.Panchaxari, D.M., Pampana, S., Pal, T., Devabhaktuni, B. and Aravapalli, A.K., 2013. Design and characterization of diclofenac diethylamine transdermal patch using silicone and acrylic adhesives combination. DARU Journal of Pharmaceutical Sciences, 21(1), p.1.